As a physics-educated college student, I don't know as much about chemistry concepts as I should have known when I made a discovery connecting J.J. Thomson's classical 1904 atomic model to the periodic table. The periodic table was present on the wall of every science classroom and lab I visited over the years -- even in high school. I didn't have a good understanding of it, however, since as a physicist we learn about electron wavefunctions, Pauli's exclusion principle, and several atom-related concepts in atomic, nuclear, and device physics. The periodic table is the most iconic thing in chemistry, but not in physics nor biology.
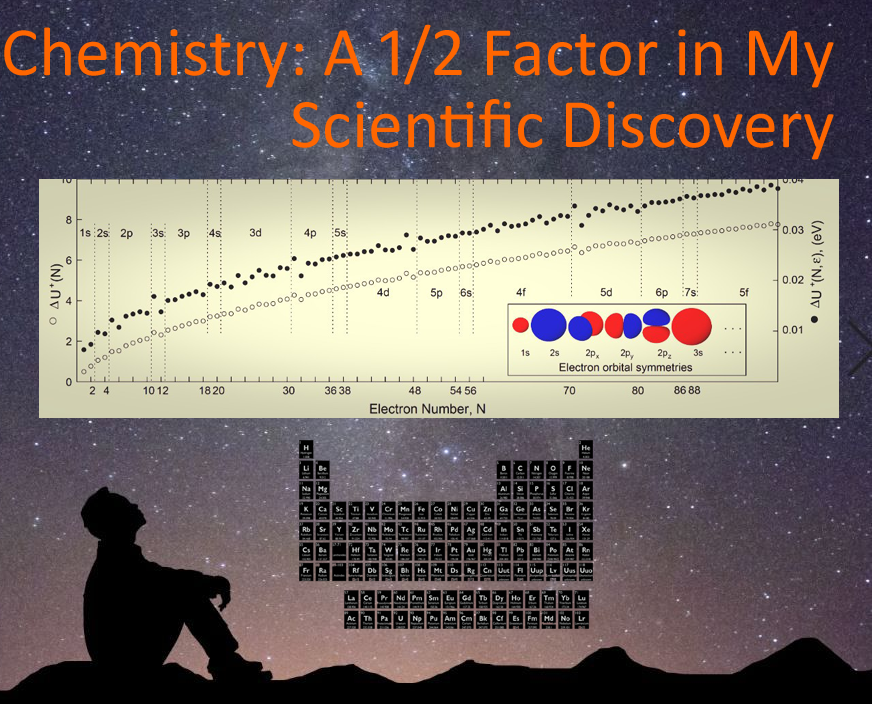
When I looked closely at a set of data in my work that turned out to be the equivalent of chemical hardness in chemistry -- the basis of the hard-soft acid-base (HSAB) model in Chemistry 101 -- I was sure something was wrong. I plotted the difference of ionization energy, I, which is the energy needed to remove an electron from an atom and affinity energy, A, which is the energy needed to add an electron to an atom. My expression was (I-A), but the chemical hardness expression first proposed by chemist R. G. Pearson was (I-A)/2. He had to be wrong, I thought, since there is absolutely no reason why that factor of 1/2 should be there! It wasn't until I looked closer into Pearson's work that I found an obscure note in a late 1990s paper where he said he included the 1/2 factor just to make the equation look like the equation for electronegativity, (I+A)/2. He also noted that chemists had already, for practical purposes, ignored his 1/2 factor for some time and thought he should admit his ad hoc inclusion of it to settle the matter once and for all. Unfortunately, word of this hasn't fully spread and many papers and textbooks include this error today.
In my work, I call this energy difference the "electron hardness." When applied to the Thomson problem of distributing electrons on the surface of a sphere, the results coincide with more things in the periodic table than I've mentioned in previous posts -- including the fact that elements larger than bismuth (83) have unstable nuclei, the hypothesized "island of stability", and the orbital sequence of the as-yet unsythesized 8th period. My discovery goes deeper than the mere shape of the periodic table.
My data set left me very wary of why the electron hardness suddenly "flat-lined" with only 83 electrons. It meant there was no geometry-based energy-preference of the system to gain an electron. The new electron had no energy-based reason to be there. It would just leave -- like radioactive decay, I thought. Protons in the nucleus. And, with some digging around, I realized bismuth is coincidentally the largest known stable element.
My undergraduate electromagnetism (E&M) professor often lamented that "E&M is riddled with errors of one half!" Here is one of them I found after making my scientific discovery.