Week of October 28, 2021
Week of October 28, 2021
In this first entry of my blog, A Piece of the Action, I want to capture the broader essence of the work. A historical context is a great place to begin, because the subject may very well have been proposed, invented, and discovered in an alternate scientific timeline that may have begun in about the early 1920s.
The Alternate Timeline
In this alternate timeline, even though the theory of quantum physics emerged triumphant in the 1920s along with revisions in subsequent years, there was much more continued enthusiasm for the old physics, or classical physics as we call it today, than in our actual timeline. This enthusiasm emerged not only because of how well the new classical models yielded features consistent with known experimental measurements of atoms in the 1920s, but they were viewed as practical contributions to the developing quantum theory. This may have very well been driven by science personalities of the time like Einstein who often exclaimed that he didn't believe the universe would leave events to pure chance as the quantum theory required, but by more deterministic mechanics that chemists might have pursued with more rigor. There was more down-to-earth philosophical debate about the underpinnings of the quantum mechanics of the day than the pseudoscientific interpretations that even today in our current timeline permeate the internet in the form of passionate half-truths, rhetoric, and other undying pseudoscientific absurdities. One aim of this blog is to hint at the down-to-earth nature of these alternate timeline contributions by presenting numerous pieces of easily verifiable connections between the classical physics frameworks of the early 1900s and natural properties of atoms found throughout the periodic table.
In particular, the work I'll present throughout the course of this blog bears much resemblance to the cubical atom model first proposed by chemist Gilbert Lewis in 1902 and published in a variety of incrementally improved versions and thoughts on the matter through about the mid to late 1920s. In a later blog entry I'll also recount my interest in tackling the nagging question I had as a doctoral student: Why didn't anyone see this before?! In an effort to answer the question I used numerical solutions of the plum pudding model published by J.J. Thomson for up to four electrons in the system, only to discover a calculation and geometry error for the four electron case. Nonetheless, using the known calculated values of the day, Thomson could have realized the connection I found in 2006.
I wondered why Thomson only calculated solutions for up to 4 electrons. Why not five? Why not six? This is when I began to realize that there wasn't an known exact solution of the Thomson problem for the five electron case until the early 2000s. (!!) So perhaps the issue was that Thomson didn't have enough numerical solutions in front of him to think about the problem in the way I did. I'll discuss my approach and thinking in another later blog entry -- in a nutshell, I was physically upset by conflicting and missing results between two areas of science. If Thomson had a modern day computer at his disposal, he and many chemists would have had more time to think about what the numerical results meant in terms of connections to known measurements on atoms. But even as I write this, I'm shaking my head in the negative because I also know that a other researchers who published their work in 1991 and 1998, respectively, did not make the connection despite having the information in front of them. In one case, the features are printed on the page but having been derived from a means appropriate to the objective of their paper and not with the objective of the periodic table in mind. They were each very much interested in other aspects of their work. There was no reason for them to give the problem as much thought as it required. My doctoral advisor had often said that he was looking for this type of connection with atomic structure since the 1980s. In his case, I tend to believe he was also much more preoccupied with other aspects of his work than the periodic table. In one published paper, he compared quantum mechanical solutions to classical solutions. This paper was exceptionally informative to me both in concept and culture. Still, I think scientists in the early 1900s had reason to think about the problem, and if they have access to a modern computer, they could have made the discovery with several numerical solutions in front of them. Again, I'll wax poetic about these things in later blogs in the hopes that my thoughts on the subject may inform others with some degree of comfort in their views and thoughts as well provide several remarks of pitfalls to avoid when attempting to think constructively about any physics problem. (Begin by erasing dogmatism from your world view.)
So, let us return to our current historical timeline here in 2021 and explore the basic ideas that have shaped things as they are today. I'll present many fun, interesting, and practical features of my work along the way that will give some passing reality to that alternate timeline that might have diverged in the 1920s, but is likely to have a far greater chance of existing and shaping our future as we introduce them today. I'll do my best to keep my eyes on the ball, but from experience I know that tunnel vision can be destructive to innovation and the spirit of discovery.
The Periodic Table as Motivation for Quantum Physics
The foundational motivation for the formulation of quantum physics was an understanding of how atoms behave both chemically and physically. Among the most important questions of the day was why does the periodic table of elements exist at all? In the early days of the 20th century, chemists largely pursued static models of electrons in atoms while physicists pursued dynamic models. Both frameworks yielded successful concepts and knowledge about atoms that remain in college textbooks today, including covalent bonding of electrons in molecules, the octet rule, quantum numbers, and electron shell-filling. I have often found it rather unfortunate that we have largely abandoned the more difficult questions of why in science -- why does the periodic table exist, for example? We often just assume that it exists and content in using that knowledge. What fundamental physical mechanisms are responsible for the existence of the periodic recurrence of chemical and physical properties of what are otherwise mere collections of subatomic particles (protons, electrons, and neutrons)? Quantum physics doesn't have a concrete answer.
For those who wonder what's the point of asking the question of why, consider that Nature doesn't provide us with all the natural resources we desire. If you want a laser that generates a particular frequency from a collection of atoms, it's very likely not to exist in Nature. So, as one of the underlying principles of metamaterial research suggests, suppose we want to manufacture a collection of meta-atoms that have their own unique periodic chemical or physical properties. How would we go about doing that? Wouldn't understanding how Nature performs the feat of periodicity be helpful? Are there periodic properties in the gate dielectric of today's smallest transistors that affect their performance? Can we introduce periodicity that might make them more robust or efficient? Knowing why there is atomic periodicity is yet another tool in the engineer's toolbox ready to be duplicated in the form of new devices and technologies.
The history of science in the 1920s and 1930s reveals that the dynamic models of atoms that ultimately resulted in what is often considered the greatest invention of humankind -- quantum physics -- quickly out-shined the static models. What more could these static models have revealed to us about the inner working of atoms if we pursued these models further? This is the question I asked myself in the weeks leading up to the defense of my doctoral thesis. I thought that perhaps if only they had modern computers to perform the calculations I did, or if only someone had parsed the problem into the pieces the way I did, then an alternate timeline could have emerged. But it didn't, so here we are considering how these things can shape the future of science and our human culture.
The Body of Work
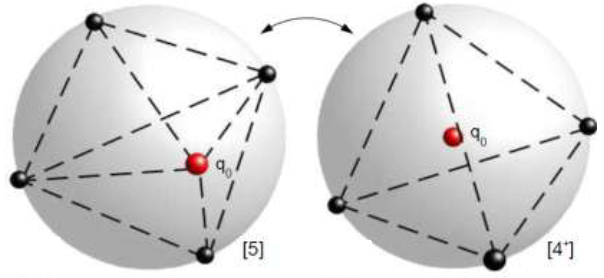
A body of personal research I continue to develop involves an inherent connection between the classical Thomson Problem, involving electrons statically distributed on the surface of a sphere, and several known properties of atoms found throughout the periodic table of elements. The Thomson problem is so-named because of its natural association with J. J. Thomson's famous 1904 plum-pudding model of the atom in which he proposed that electrons (that he called "corpuscles" at the time) arranged themselves in static configurations inside a uniformly distributed sphere of positive charge. The Thomson problem is a natural consequence of the plum pudding model. The Thomson problem has become a valuable benchmark for today's electromagnetism simulation apps, but it has never yielded any connection with Thomson's original intent of his model: atomic structure and the periodic table. That is, until now.
In a future blog entry I'd like to recount the fun chain of coincidences and conversations that led me to this discovery. In simple terms, as I reflect on the hows and whys I realize my model is the plum pudding model except I used an electrically neutral dielectric sphere in place of Thomson's positively charged spherical region of space. Why did I do this? I was modeling a spherical quantum dot -- also known as an artificial atom.
In the last several years I have identified numerous connections, both salient and subtle, involving this deceptively simple geometric problem that today has no general mathematical solution(!). It's a special form of the 7th "challenge problem for the 21st century" posed by mathematician Steve Smale. Smale's list includes eighteen challenge problems you may find of interest here. For more information about the Thomson problem, see this Wikipedia page to which I've contributed in the past.
|
|
| The amount of energy needed to move an electron from the surface of a sphere to its origin for any geometric N-electron solution of the Thomson problem, is proportional to the first ionization energy of the corresponding atom on the periodic table. See this Journal of Electrostatics article concerning this charge transformation or the freely-accessible Arxiv version. |
How lovely it would be to establish an intimate mathematical connection between what we as scientists today consider "quantum mechanical" phenomena, such as electron shell-filling, ionization, and chemical bonding, and the alluring beauty of basic geometric arrangements of electrons on a sphere. How fundamentally important is geometry to all of science? How would this connection change our thinking, teaching, and practice of science?
How much more will we learn by pursuing the details of this connection?
Go Nuclear
These geometries apply to electrons in atoms, but do they also apply to protons in the nucleus of atoms? Electrons aren't adjacent to other particles inside an atom. Instead they're bound to the nucleus by electromagnetic forces at a distance. Protons, however, are adjacent to other particles in the nucleus -- neutrons. But are there any connections to be made between the Thomson problem and collections of protons in the nucleus? That's the subject of next week's blog entry.
Read More:
"Discrete transformations in the Thomson Problem" (2014) at Journal of Electrostatics or Arxiv version.
Smale's Problems on Wikipedia