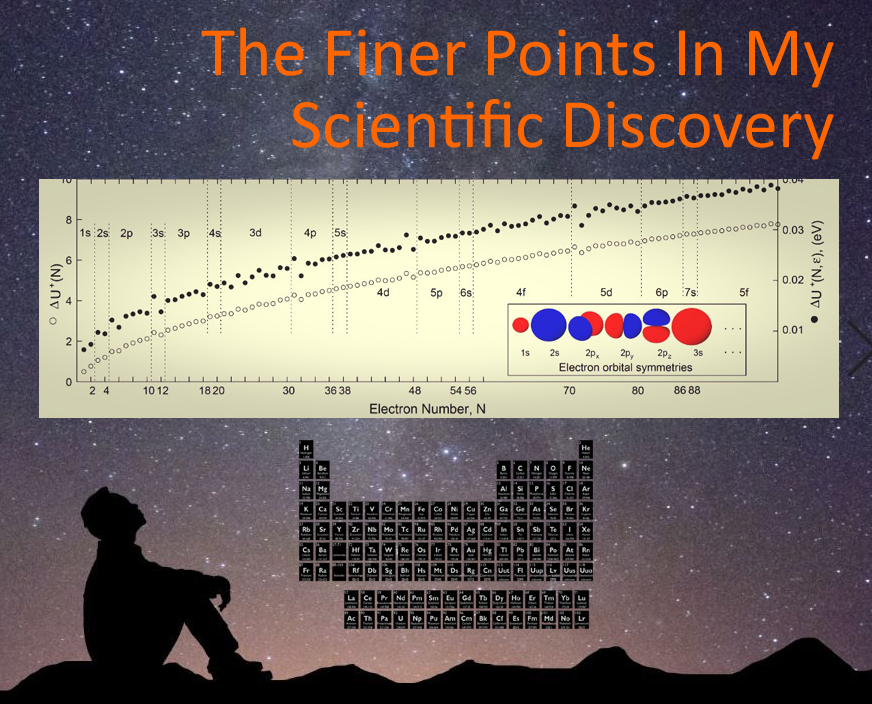
In the picture attached to this post, you see my classical electrostatic energy plotted with vertical lines separating the familiar electron orbitals of outermost electrons in atoms throughout the periodic table (i.e. 1s, 2s, 2p, . . .). The upper data is from electrons in a dielectric sphere. The lower is from electrons in free space.
Let's walk through a few of these.
Notice abrupt energy jumps at these divisions, for example, between 1s and 2s in which the size of these sphere-shaped orbitals increases. Another abrupt jump occurs between 2s and 2p. Here, the actual orbital shape changes from a sphere (2s) to a dumbbell (2p)! There's another jump two data points into the 2p orbital. Is this because there are three unique dumbbell-shaped orbitals along three axes (x,y,z). That is, 2p_x, 2p_y, and 2p_z. That's one of the many finer points of this work. Does this help build your confidence in this work?
These are the first few data points that got me excited. I only had 7 data points at first and knew what I was looking at. Previous data plots had no features like these whatsoever. For some reason, my doctoral advisor wasn't excited by them . . . yet.
Moving forward, with every data point I calculated, I eagerly waited to see my data "do another trick" -- as I called it because it was kind of like watching my calculations perform magic tricks and I was a bit beside myself with every correspondence with the periodic table I observed. A jump to 3s (back to a spherical orbital!). Another to 3p (back to a dumbbell shaped orbital!). Then another to 4s (sphere again!).
How exciting! No one knew this but me.
That was the first month. I reviewed it with my advisor. He began to warm up to my work.
I looked back to the periodic table and realized I hadn't yet calculated anything for a jump to a different shaped d-orbital. When I did the calculation, this was the first time I was disappointed. I triple and quadruple checked my work. I didn't know why there was no jump between 4s and 3d. I'm not a chemist, so it took a while to recall that the energy of 4s electrons is known to be very close to 3d electrons, and that this is a large part of why atoms in the 3d region of the periodic table (the middle of the 4th row, or period) contain electrons that violate what are called "shell-filling rules". These rules tend to predict which orbitals are occupied by each electron as you "build" up the electron structure. We call this "aufbau", a German word meaning "to build or construct". It's also why, I reasoned, there is not an abrupt jump in my data set.
Aha! Another VERY fine correspondence!
There are so many more finer points like these. Some include those shell-filling rule violations. Many I haven't yet identified.
My certainty in the value and credibility of this work is solidified with every new finer correspondence.