As I've noted elsewhere, my discovery of numerous correspondences between the classical problem of distributing electrons on a sphere -- the Thomson problem -- and the entire periodic table, was accompanied by other theses in my PhD dissertation. Among these was my electrostatic interactions framework of excess electrons in a dielectric material.
Without these other theses, it's unlikely I'd have made a discovery. I was happily swimming in these subjects, having made novel contributions to them all, when I noticed a pattern of energies that fits the distribution of orbitals in the periodic table.
The first citation of my work was to point to it as the reason why electrons naturally move to the surfaces of materials. The key is minimized energy and repulsion of like charges.
A friend of my doctoral advisor whose career was spent trying to find an exact solution for the helium atom said I got much closer to a solution than he ever did. I happened to see a note on my advisor's desk one day saying his friend wanted to hire me but that, having just retired, he had no funds. I recently looked him up only to discover he passed away in 2021.
Another friend of my doctoral advisor, a computational biologist, originally thinking my model was my advisor's work, said he was surprised a physicist would include a particular energy term. He said that without this term, his computational models wouldn't agree with real biological phenomena. My advisor confessed it was my work and not his.
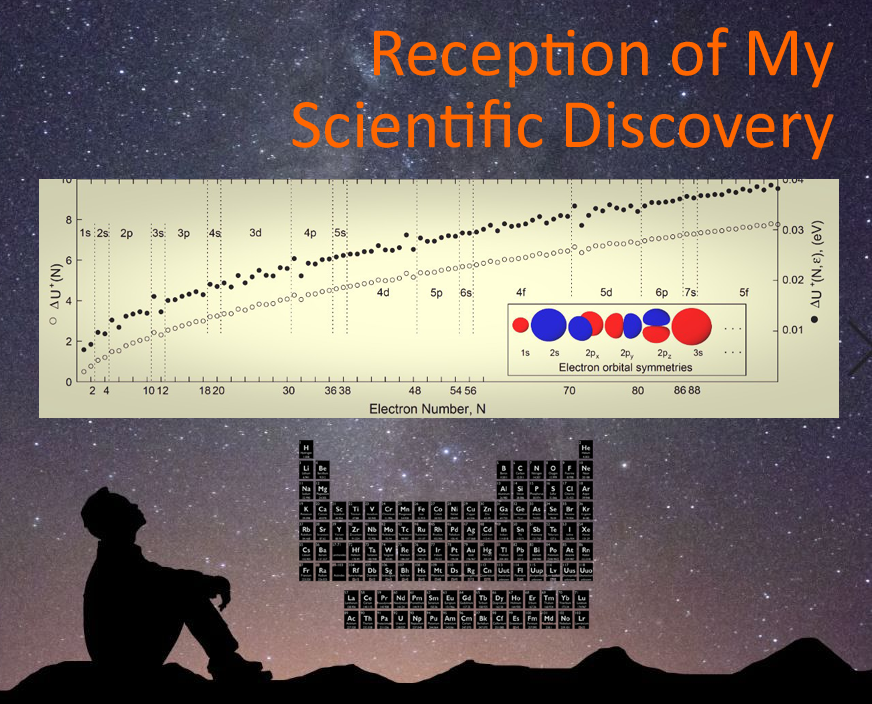
An "ab initio calculator," as my advisor liked to call a certain group of physicists, quickly dismissed my discovery saying "it's been done before." He claimed we "cheated" in an early paper comparing my classical energies to real ionization energies using quantum mechanical atomic radii. He wasted his postdoc's time calculating and plotting atomic radii to prove his point. What *is* known that quantum mechanical radii have been done before What hadn't been done before was applying my classical framework to atomic ionization. I now compare my data to size-independent empirical atomic ionization energy data -- and discovered yet another intimate connection with the broader periodic table! Thanks for the motivation, ab initio calculator. Making this new connection only added more weight behind my advisor's way of introducing me to other people as "my deepest thinker."
Of great curiosity to me, over the years I've noticed that anyone who looks closely at my work tends to see something in it related to their own scientific background. After all, the periodic table lists the fundamental components of all "normal matter" in the universe, and my scientific discovery is a very rudimentary new piece of the science puzzle that's incredibly simple to obtain. I have often suggested that my work "may put a rug under quantum mechanics."
You're likely to find something useful in it.