Scientific discoveries are often made by accident. The discovery I made about atomic structure wasn't accidental. I had all the calculations done and ready to go, but it wasn't until I *literally* became physically ill with confusion of how others thought about the problem that I knew what to with my calculations. Let me explain.
First, I couldn't understand why almost every paper on the subject treated it with quantum mechanics and not classical mechanics. Of the very few that used classical mechanics, none reported interesting correlations with known abrupt geometric symmetry differences between say the 12 and 13 electron systems. With such large geometric differences, surely someone reported corresponding energy differences. It must be somewhere in my data, I thought.
Second, I realized that the energy differences others considered were muddied because they depended on both changing the geometric symmetry and the number of electrons (gain or lose an electron). So I devised a way to keep the number of electrons the same and only change the geometry. Late one Sunday night I realized I already had the calculations needed to check my approach.
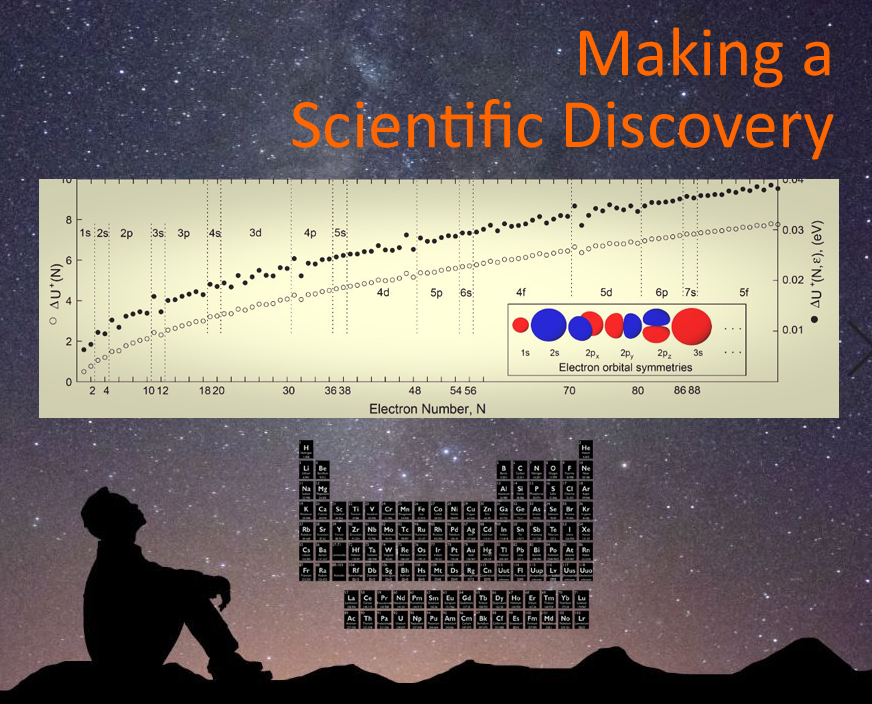
From these evaluations emerged something absolutely bizarre. Instead of very gradual changes in every energy plot I had done with my classical mechanics framework, this one had big, abrupt jumps and drops. What were these? (See picture.)
The third piece of the puzzle came into place when I looked at a periodic table on my wall. I previously used it to help think about semiconductor material properties during my masters degree program, but now I had something far more fundamental to think about: the physical origin of electron orbitals.
I noticed that for every complete electron orbital there was an energy jump in my data. For the top row of the periodic table, there are two electrons that complete not only an orbital, but a shell (hence, the full first row); jump! The next two atoms have two more electrons that complete an orbital; jump! Then two more electrons complete an orbital; jump! I only had 7 data points on my plot, but I knew I just discovered a fundamental electrostatic property of the entire periodic table just as the physicist J. J. Thomson had hoped to find with his 1904 classical model of the atom.
I rushed to my advisor's office. He must have thought I had lost my mind, because he sent me off to do busy work based on his ideas on how to do the problem. Sure of what I discovered, I chose instead to disappear for a couple weeks, do more calculations to show that the data would "jump" at more orbitals on the periodic table -- especially between the 12th and 13th electron systems that started my thinking on the right path.
A month later my advisor said he was looking for this kind of connection since the 1980s just as others were. My thinking was clearly different from theirs.