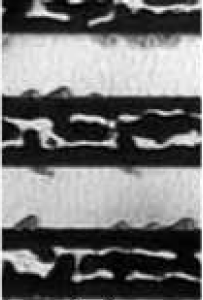
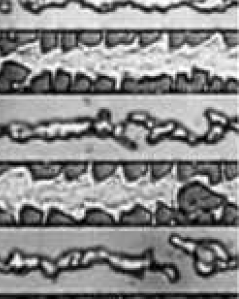
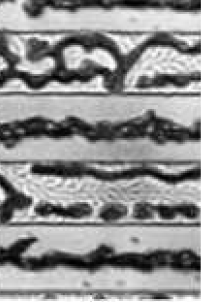
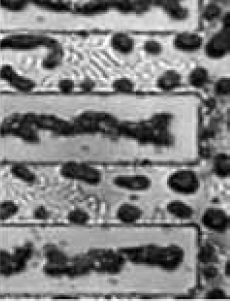
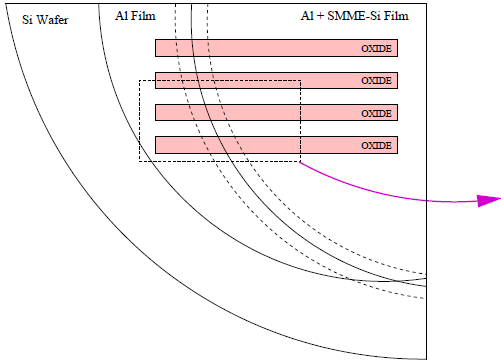
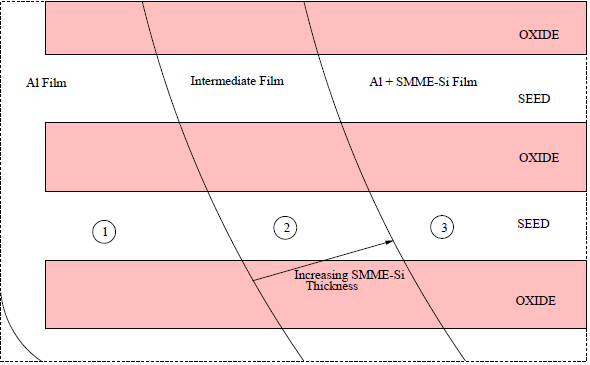
SEM micrographs and optical images were acquired to investigate the surface morphology of several substrates. Fig. 5.14 provides a broad optical overview of Al island formation on oxide during the SMM-SOI process. Fig. 5.15 is an SEM study of the same 10/10 $\mu$m region (cf. Fig. 4.2) studied in Fig. 5.14. Such studies exploit the cross-patterned nature of the Si and Al deposition areas (Fig. 5.16) formed by virtue of the sample heater/holder assembly geometry shown in section 4.5.
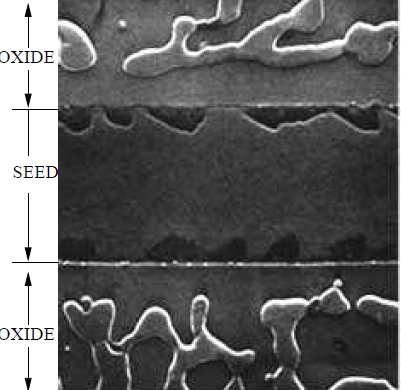
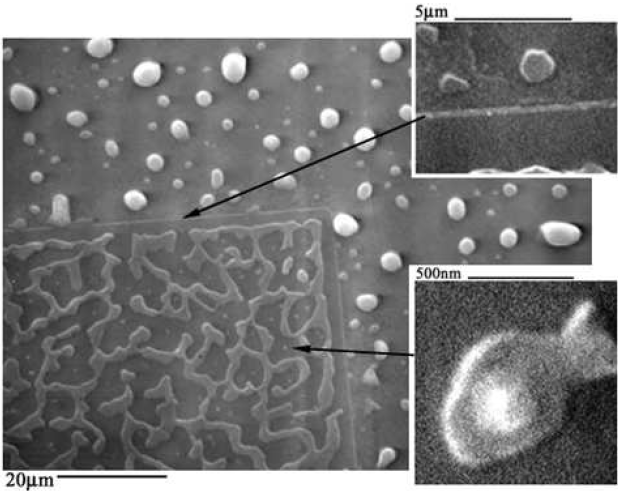
The Al-only region (Figs. 5.14a,b and 5.15a) shows the film beginning to separate from the oxide walls. In the optical image (Fig. 5.14a,b), the Al film is bright. The underlying Si substrate is dark. In the SEM image (Fig. 5.15a), the Al film is somewhat brighter than the underlying Si substrate due to the availability of free electrons in the metal Al film. On the oxide region, the bright Al is shown to readily conglomerate into elongated islands along the length of the oxide edges.
In Figure 5-14c, the Al film in the seed area continues to separate from the oxide walls, and the elongated Al islands on oxide begin to align lengthwise along the center of the oxide patterns. Both the Al film in the seed area and Al islands on oxide begin to show considerable damage produced by the diffusion of Si.
During silicon deposition (Figs. 5.14d,e and 5.15c), the Al film in the seeding area transforms into elongated islands near the edge of the oxide walls on either side of the seed area and finally separates into small islands of Al along the the oxide walls. On oxide, the elongated Al islands continue to become aligned along the center of the oxide strips and are damaged during Si diffusion. The severity of the damage is evident in the darkening of the Al islands in the optical image and the roughness of the SEM micrograph.
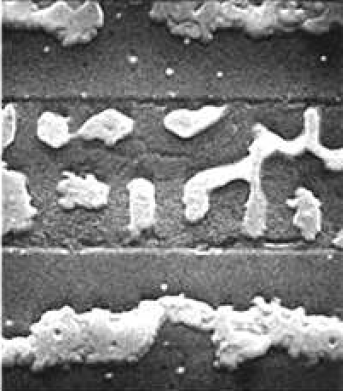
Cross-sectional SEM (Fig. 5.9) of the 2/2 $\mu$m oxide pattern shows a considerable length of the oxide pattern covered with a smooth film of Si. On the 10/10 $\mu$m pattern examined in Figures. 5.14 and 5.15, the 2/5 $\mu$m region in Figure 5.18, the 5/10 $\mu$m region in Figure 5.19, and the 50/500 $\mu$m region of Figure 5.17, along the edge of the oxide, there are smooth regions of a few microns along the entire length of the oxide strip. This oxide-long phenomenon may be explained as a separation of the Al film from the oxide edge. This separation occurred prior to Si deposition, however, as shown by the 50/50 $\mu$m region of Figure 5.17, the Al film over oxide is separated by the presence of small contaminants. Overall, all oxide patterns demonstrate similar Al film characteristics in the oxide regions.
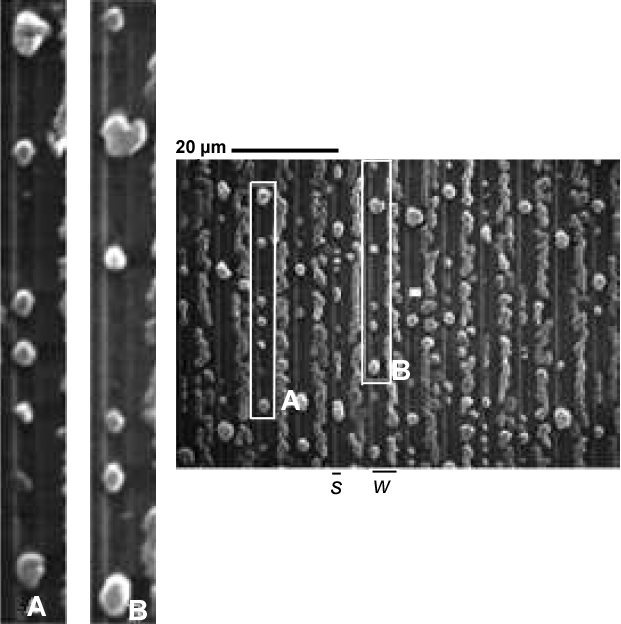
In the optical/SEM study of the 10/10 $\mu$m region in Figsures 5.14 and 5.15, Al islands are found to be two separate chains along both sides of the oxide pattern. These two chains coalesce into a single chain of islands along the center of the oxide strip as Si is deposited. An explanation for this is the advancement of the SMME growth front laterally over the oxide that may push Al islands toward the center of the oxide strip.
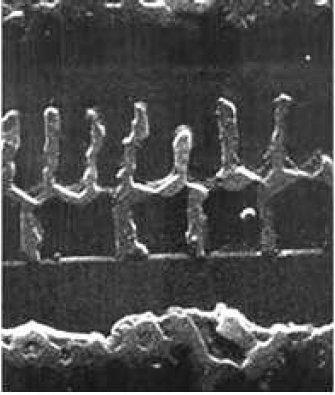
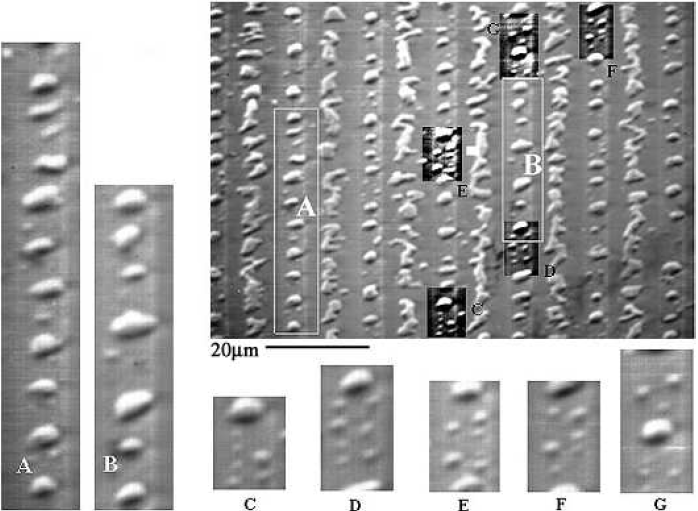
In the seed regions, a similar formation of Al island patterns is evident on all oxide patterned regions. Typically, small Al islands form 1-2 $\mu$m(cf. insets C-G of Fig. 5.19) and larger Al islands appear $\gt2\mu$m, from the oxide walls. The size and location of these islands may be attributed to the nucleation of the Al film, having been strongly affected by the diffusion of Si. Note that the Al film by itself(Fig. 5.15a), that has undergone the same stages of annealing as the rest of the sample, remains intact aside from the separation of the film from the oxide walls. This suggests that native oxides in the seed regions do not necessarily initiate the formation of Al islands, but that the diffusion of Si provides a mechanism that exploits the Al film separation from oxide walls, causing the Al film to nucleate into the observed islands. These distinct Al island sizes, shapes, and locations represent an interesting phenomenon that may be unique to the SMM-SOI process and merit further investigation.