The diffusion of atoms in a solid metal is important to a better understanding of the solid-metal-mediated epitaxy (SMME) process in order that the process may be adapted for use with other materials and in conjunction with other technologies. Here a derivation of Fick's second law[1] of diffusion is provided by considering a one-dimensional state of flux and conserving mass. An analytical solution of the diffusion equation is obtained and later used to illustrate the process based on specific parameters for the Si-Al system investigated.
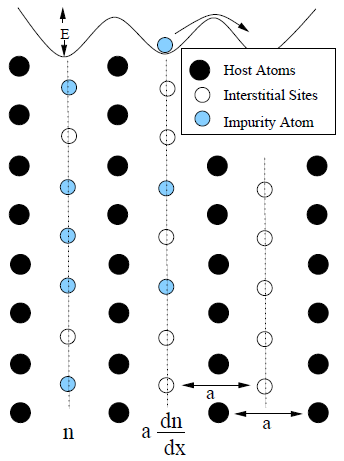
To diffuse, an impurity atom is required to overcome the potential energy barrier, \(E\), of a neighboring host atom. For simplicity, consider two parallel planes of diffusing atoms separated by some distance, \(a\). On the first plane, there are \(n\) atoms; on the next there are \(n+a\frac{\textrm{d}n}{\textrm{d}x}\) as shown in Figure 1.8a. Diffusors on one plane acquire sufficient energy to overcome the potential barrier, \(E\), during some fraction of time, \(\exp(-E/k_b T)\), and pass to the neighboring plane of interstitial sites.
Figure 1.8: Simplified Construction of Diffusion System. a) Number of impurity atoms on adjacent (parallel) interstitial planes. Note: Impurity atoms must overcome the potential energy barrier, \(E\), to pass a host plane and diffuse to a neighboring interstitial site. b) An ideal one-dimensional system for the flux of atoms between planes \(x=X_1\) and \(x=X_2\). |
If the diffusors vibrate with some frequency, \(\nu\), they have a probability of overcoming the barrier,
| $$p=\nu \exp \left( \frac{-E}{k_b T} \right)$$, | (1.1) |
called the jump frequency of the atom. Here, the activation energy, \(E\), must be overcome at some absolute temperature, \(T\).
The net number of atoms, \(N\), passing one diffusor plane to the next in some unit of time is then given by
$$N\approx-pa\frac{\textrm{d}n}{\textrm{d}x}$$.
where the negative sign indicates diffusion in a direction opposite the concentration gradient. Consider a concentration, \(C\), of diffusors in some unit area of a given plane, \(n=aC\). The net flux of diffusors between planes may be expressed as
| $$\begin{array}{ccl} J & \approx &-pa^2 \frac{\textrm{d}C}{\textrm{d}x}\\ &\approx &-va^2\exp\left( \frac{-E}{k_b T} \right) \frac{\textrm{d}C}{\textrm{d}x}\\ &\approx & -D\frac{\textrm{d}C}{\textrm{d}x} \end{array}$$. | (1.2) |
Here, \(D\) is the diffusion constant, or diffusivity. The negative sign indicates that the flux, \(J\), is in the direction of decreasing concentration gradient.
The condition of the conservation of mass must also be satisfied. Consider a one-dimensional system as shown in Fig. 1.8b. Such a system can be approximated if the off-axis dimensions are impermeable to diffusors. The only sources of mass in the system are through planes \(X_1\) and \(X_2\), each of area \(A\).
The total mass in this volume is given by
$$M=\int_{X_1}^{X_2}C(x,t)A\textrm{d}x$$.
The time-dependent change in this mass, \(\frac{\textrm{d}}{{\textrm{d}t}}M\), is then given by
| $$\frac{\textrm{d}}{\textrm{d}t}\int_{X_1}^{X_2}C(x,t)A\textrm{d}x=J(X_1,t)A-J(X_2,t)A$$. | (1.3) |
The area, \(A\), of the cross section vanishes. The result of the integral depends only on time and is therefore an ordinary derivative. However, \(X_1\) and \(X_2\) are held fixed, then
| $$\frac{\textrm{d}}{\textrm{d}t}\int_{X_1}^{X_2}C(x,t)\textrm{d}x=\int_{X_1}^{X_2}\frac{\partial}{\partial x}C(x,t)\textrm{d}x$$. | (1.4) |
If the flux, \(J\), is assumed to be continuously differentiable between \(X_1\) and \(X_2\), then
| $$(J(X_1,t)-J(X_2,t)=-\int_{X_1}^{X_2}\frac{\partial J}{\partial x} \textrm{d}x$$. | (1.5) |
With (1.4) and (1.5), (1.3) becomes
$$\begin{array}{rcl} \int_{X_1}^{X_2}\frac{\partial C(x,t)}{\partial t}\textrm{d}x+\int_{X_1}^{X_2}\frac{\partial J(x,t)}{\partial x}\textrm{d}x&=&0\\ \int_{X_1}^{X_2}\left( \frac{\partial C(x,t)}{\partial t}+\frac{\partial J(x,t)}{\partial x}\right)\textrm{d}x&=&0 \end{array}$$
that gives the condition of conservation of mass,
| $$\frac{\partial C(x,t)}{\partial t} = -\frac{\partial J(x,t)}{\partial x}$$. | (1.6) |
Combining (1.2) and (1.6),
| $$\frac{\partial C(x,t)}{\partial t}=\frac{\partial}{\partial x}\left[ D\frac{\partial C}{\partial x}\right]$$. | (1.7) |
If \(D\) is held constant, then Fick's second diffusion equation is obtained[2],
| $$\frac{\partial C(x,t)}{\partial t} = D\frac{\partial^2 C}{\partial x^2}$$. | (1.8) |
This equation can be analytically solved for only a few real systems. An idealized solution will be considered in the next chapter for the investigated system of materials with relevant boundary conditions.
References
- , Introduction to Solid State Physics, 7th ed. Wiley, 1996.
- , VLSI Technology. Bell Telephone Laboratory, Inc., 1983.